In a world where clean, safe drinking water is more crucial than ever, Reverse Osmosis (RO) technology stands out as a game changer. Imagine having access to pristine water at the turn of a tap—RO makes this a reality by transforming ordinary tap water into a crystal clear, contaminant free elixir. But how does this remarkable process work? And why is it considered one of the most effective methods for purifying water? Join us as we dive into the science behind RO technology, exploring its benefits, applications, and why it's becoming an essential fixture in homes and industries alike.
History
In 1748, Jean-Antoine Nollet first observed the process of osmosis through semipermeable membranes in a laboratory. Using a pig's bladder as the membrane, he demonstrated that a solvent could selectively pass through this semi-permeable barrier due to natural osmotic pressure. The solvent would continue to move through the membrane until equilibrium was achieved on both sides of the bladder.
Reverse Osmosis Through the Years
For the next 200 years, osmosis remained a laboratory curiosity. It wasn't until 1949 that researchers at the University of California, Los Angeles, began exploring osmosis and semipermeable membranes as a means to desalinate seawater. In the mid-1950s, both the University of California and the University of Florida successfully created fresh water from seawater, but the technology was not commercially feasible due to high flux rates. John Cadotte of FilmTec Corporation later pioneered a breakthrough by developing membranes with high flux and low salt passage through interfacial polymerization of m-phenylenediamine and trimesoyl chloride. By 2001, more than 15,000 desalination plants had been established or were in planning stages globally.
In 1977, Cape Coral, Florida, became the first U.S. city to implement large-scale reverse osmosis, starting with a capacity of 3 million gallons per day. By 1985, the city had expanded to operate the world's largest low-pressure reverse-osmosis plant, producing 15 million gallons per day, thanks to its rapid population growth.
What is Reverse Osmosis?
Reverse osmosis involves applying pressure greater than the osmotic pressure to force a solvent from an area of high solute concentration through a semipermeable membrane to an area of lower solute concentration. The most significant and widely recognized use of reverse osmosis is in desalinating seawater and other brackish waters. In this process, seawater or brackish water is pressurized against one side of the membrane, which drives the movement of salt-free water through the membrane, resulting in purified water on the low-pressure side.
How does Reverse Osmosis works?
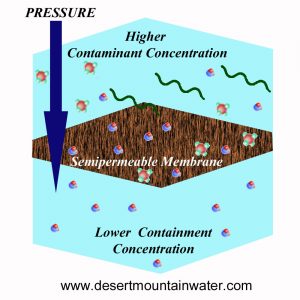
Reverse Osmosis, sometimes referred to as hyperfiltration, was first developed over 52 years ago with the creation of a successful semi-permeable membrane. This membrane selectively allows water molecules to pass through while blocking contaminants from reaching the clean side, known as the permeate side. In the early 1970s, the first commercial low-pressure semi-permeable membrane was introduced, capable of producing 1 to 5 gallons of clean drinking water per day. Today, advancements in technology have significantly increased the capacity of such low-pressure household systems, allowing them to produce up to 100 gallons per day or more ample for a family's drinking and cooking needs. Many cities and water authorities have invested heavily in this technology for municipal water recycling programs, especially as natural water sources become more unreliable. Reverse osmosis has proven to be both advanced and dependable.
Principles of Reverse Osmosis
Water naturally moves from a region with lower saline concentration to one with higher saline concentration, a phenomenon known as osmosis. An example of this is when plant roots absorb water from the soil.
Reverse osmosis, on the other hand, is the reverse process. By applying pressure, water is pushed against the natural osmotic flow, moving from an area of higher saline concentration to one of lower concentration. Reverse osmosis membranes have incredibly small pores, measuring just 0.0001 microns—about one millionth the diameter of a human hair. Since bacteria and viruses are approximately 5000 times larger than these pores, only water molecules can pass through. As a result, contaminants such as particulate matter, heavy metals, and pesticides are filtered out and removed with the concentrated wastewater.
Conclusion
In summary, reverse osmosis is a transformative technology that harnesses the principles of osmosis in reverse to deliver highly purified water. By applying pressure to force water through a semi-permeable membrane with pores smaller than many contaminants, this process effectively removes impurities such as bacteria, viruses, heavy metals, and pesticides. From its initial development over five decades ago to its current advancements, reverse osmosis has become a crucial solution for producing clean, safe drinking water. Whether in household systems or large-scale municipal water recycling programs, this technology continues to play a vital role in ensuring access to pure water and addressing the challenges of unreliable natural water sources. Its proven reliability and efficiency make reverse osmosis a cornerstone of modern water purification.
SANAKY is one of the firms in producing RO water purifiers in Vietnam, offering a diverse range of models renowned for their quality and reliability. Trusted by numerous families and businesses alike, our products cater to various needs.
For further assistance in selecting the right product or for any inquiries, please feel free to leave a comment below or reach out to us via the provided email or phone number.
 Vietnamese
Vietnamese  English
English  Chinese
Chinese  French
French  Spanish
Spanish  Russian
Russian  Arabic
Arabic  Portuguese
Portuguese